Contact : Laurent El Kaïm, professor
Isocyanide and multicomponent reactions (MCR)
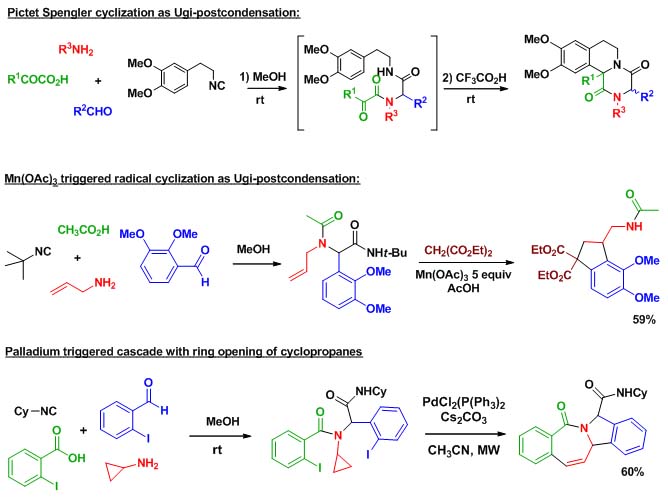
MCRs represent an important trend in organic synthesis. These reactions, that assemble more than two components within a same molecular framework, have attracted considerable interest in relation with the structural complexity reached in a reduced number of synthetic steps. Indeed organic chemists, in response to growing environmental concerns, are more and more adapting their synthetic strategies in order to comply with the concepts of step and atom economy. In this context, multicomponent reactions are important tools. When MCRs involving more than three components are considered, isocyanide chemistry is often introduced due to the high efficiency and versatility of the Ugi 4-component reaction. This reaction discovered in 1959 has been extensively used in the last twenty years. Most 4-component synthetic strategies display a Ugi coupling followed by a so-called "post-condensation" step in order to form highly diverse scaffolds in less than three steps. Research on isocyanide based MCR at ENSTA has focused on original Ugi post-condensations involving complex cascades, new organometallic catalyzed process as well as radical couplings.
Scheme 1 presents two examples taken from our research :
Scheme 1 : Ugi post-condensations
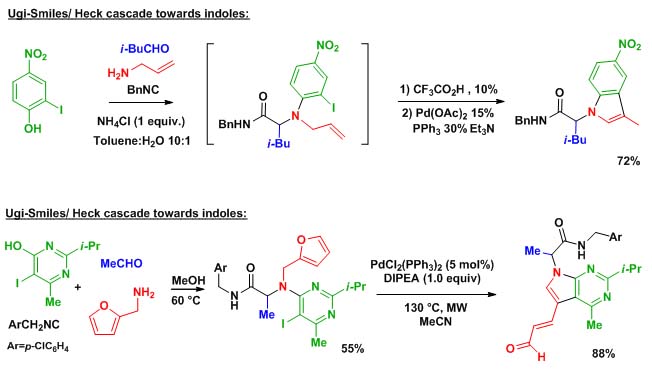
On other important strategy in our group is to think about original ways of introducing isocyanides in multicomponent couplings. Among various projects involving Nef isocyanide reaction, [4+1] cyclizations, and variations on the Ugi coupling, our Ugi-Smiles extension certainly represent the project with the highest synthetic potential. Taking phenols and hydroxy-heterocycles as acidic partners in Ugi reaction, we were able to disclose a new N-arylative coupling involving as Smiles rearrangement as the key step.This new family of Ugi couplings opens the way to many post-condensation studies involving new cyclocondensations as well as organometallic couplings.
Scheme 2 represent a selection of these studies :
Scheme 2 : Ugi-Smiles post-condensations
Organometallic chemistry
During our attempts to perform aldol type addition between hydrazone and aldehydes, we discovered that the addition of an aldehyde to the preformed complex of trimethylaluminum and hydrazone lead to an N-alkylated compound with transfer of the methyl group. Apart from the mechanistic interest of this reaction (analogous reaction with secondary amine instead of the hydrazone is not described), this coupling allows the preparation of highly hindered hydrazine derivatives...
>>> Read more...
Hydrazone chemistry
We have been interested in hydrazone chemistry for a long time, and various axes have been explored. For instance, we have developed a new Mannich-type coupling involving hydrazones as the nucleophilic partner. Based on the former Keil and Reid work (1965), we developed a very effcient thre-component access to a-aminohydrazones which are stable precursor of azoalkenes. The use of N-benzyl piperazine allows efficient couplings whatever the aldehyde used and some mild conditions for the azoalkene generation. These heterodienes could be trapped in various cycloaddition reactions -[4+1] and [4+2]- to form different heterocyclic scaffolds...
>>> Read more...
Isocyanide chemistry
Nef-type reactions : Trifluoroacetic anhydride
Isocyanides addition to trifluoroacetic anhydride allows a straightforward preparation of trifluoropyruvamides derivatives. These compounds are useful fluorinated synthetic intermediates ; we have demonstrated that various nucleophiles are easily added on these stable hydrates...
>>> Read more...